Hydrogen sulfide is slightly soluble in water and acts as a weak acid pk a 6 9 in 0 01 0 1 mol litre solutions at 18 c giving the hydrosulfide ion hs also written sh.
Hydrogen sulfide state at room temperature.
Chemical physical and thermal properties of hydrogen sulfide.
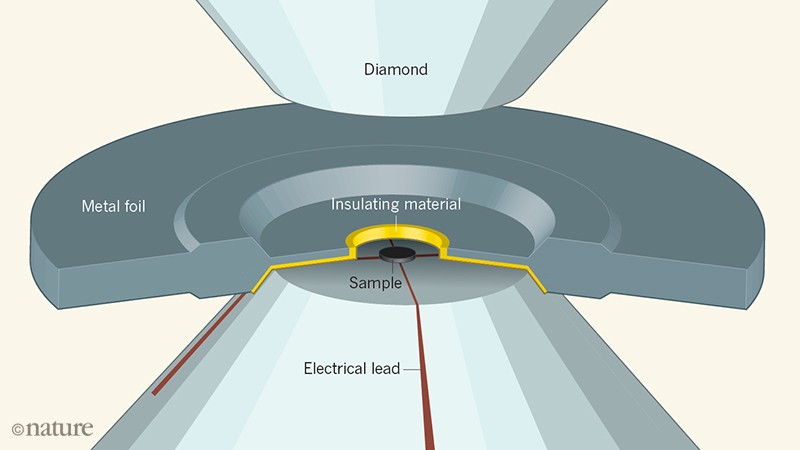
Numerous groups have claimed to make such metallic hydrogen using diamond anvil cells palm size devices in which a target substance gets crushed to.
What is the oxidation state of the noble gas in each of the following.
When exposed to air it slowly oxidizes to form elemental sulfur which is not soluble in water.
H2s is in gaseous state of matter at r t.
These chemicals were placed on a diamond anvil and compressed then exposed to a.
Hydrogen under intense pressure.
Hydrogen sulfide and its solutions are colorless.
Hydrogen sulphide contains the element hydrogen in a 1 oxidation state and the element sulphur in a 2 oxidation state.
In 1968 neil ashcroft a theorist at cornell university had suggested a different type of material should display bcs superconductivity above room temperature.
Whether a compound will be a solid liquid or gas at a given temperature can be explained by the attractive forces between its molecules.
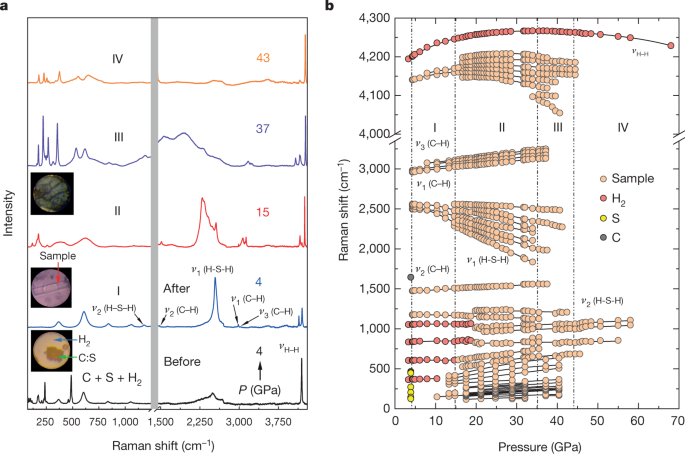
The research team used sulfur and carbon then added hydrogen forming hydrogen sulfide h 2 s and methane ch 4.
In 1968 neil ashcroft a theorist at cornell university had suggested a different type of material should display bcs superconductivity above room temperature.
Explain why hydrogen sulfide is a gas at room temperature whereas water which has a lower molecular mass is a liquid.
The water molecule has a stronger dipole a negatively and.
Hydrogen sulfide becomes superconductive under high pressure at minus 70 degrees celsius.
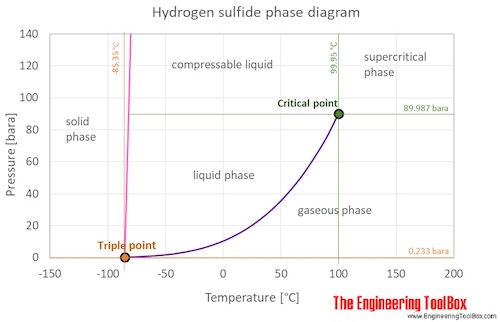
The phase diagram of hydrogen sulfide is shown below the table.
Hydrogen sulfide is used in the manufacture of chemicals in metallurgy and as an analytical reagent.
Chemistry q a library explain why hydrogen sulfide is a gas at room temperature whereas water which has a lower molecular mass is a liquid.
By max planck society.