The gas can also cause blindness by rapid destruction of the corneas.
Hydrogen fluoride state at room temperature.
It is a gas and that would be the state of matter.
Its chemical formula consists of one hydrogen atom and one fluorine atom giving it the formula hf.
The boiling point of hf if 19 5 c while that of hcl is 85 c.
Gaseous weight of hydrogen fluoride at room temperature is therefore probably a mixture of.
And can hcl form hydrogen bonding.
Being ionic compounds they are solid at room temperature.
At room temperature the molar volume of hydrogen fluoride has a mass of about 5 0 g.
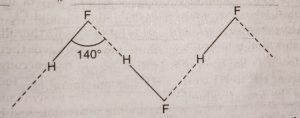
Hydrogen fluoride has a very strong permanent dipole and so it forms stronger permanent dipole permanent dipole forces with other hf molecules.
110 carbenium ion type rearrangement and elimination appear less problematic with dast than with hf reagents.
It gives fluoroalkanes from alcohols at lower temperatures 50 to 78 c than sf 4 and is safe below 40 c.
Fluorine the element from which the fluoride ion is derived is a gas at room temperature.
Hydrogen fluoride is a highly dangerous gas forming corrosive and penetrating hydrofluoric acid upon contact with moisture.
See full answer.
Hydrogen fluoride has a molar mass of 20.
What state of matter does hydrogen have in room.
Is hydrogen fluoride liquid at room temperature while hydrogen chloride is gaseous.
Above room temperature hydrogen fluoride is a gas but can be condensed into solid crystals when the temperature is lower than roughly 20 degrees celsius.
Why te compounds of hydrogen and fluorine is a liquid at room temperature on cold day when the other hydrogen halides are gases.
However hydrogen is not solid at room temperature.
If it were a solid at room temperature then that would be the state of matter.
In a cool room yes.
The formula weight of hydrogen fluoride is 2 0.
Hydrogen fluoride boils near room temperature much higher than other hydrogen halides.